Advancing Nanoscale Control of Material Surfaces and Interfaces for Electrified Chemical Processes – Enabling Efficient and Innovative Energy, Fuel, and Chemical Solutions
Electrochemical reactions – chemical reactions powered by electricity, and those that generate electrical energy – offer innovative, efficient ways to produce and use energy, fuels, and essential chemicals and materials (Fig. 1). They create new pathways to utilize abundant resources for producing energy and chemicals, such as splitting water to generate H2 and converting CO2 to hydrocarbons. Operating near ambient temperatures and pressures, electrochemical processes can achieve higher overall energy efficiency than conventional methods. They also enable decentralized operation with enhanced flexibility and rapid response times, allowing for increased productivity wherever and whenever needed. Electrochemical reactions have the potential to transform how we produce and utilize energy, fuels, chemicals, and materials with greater efficiency and security.
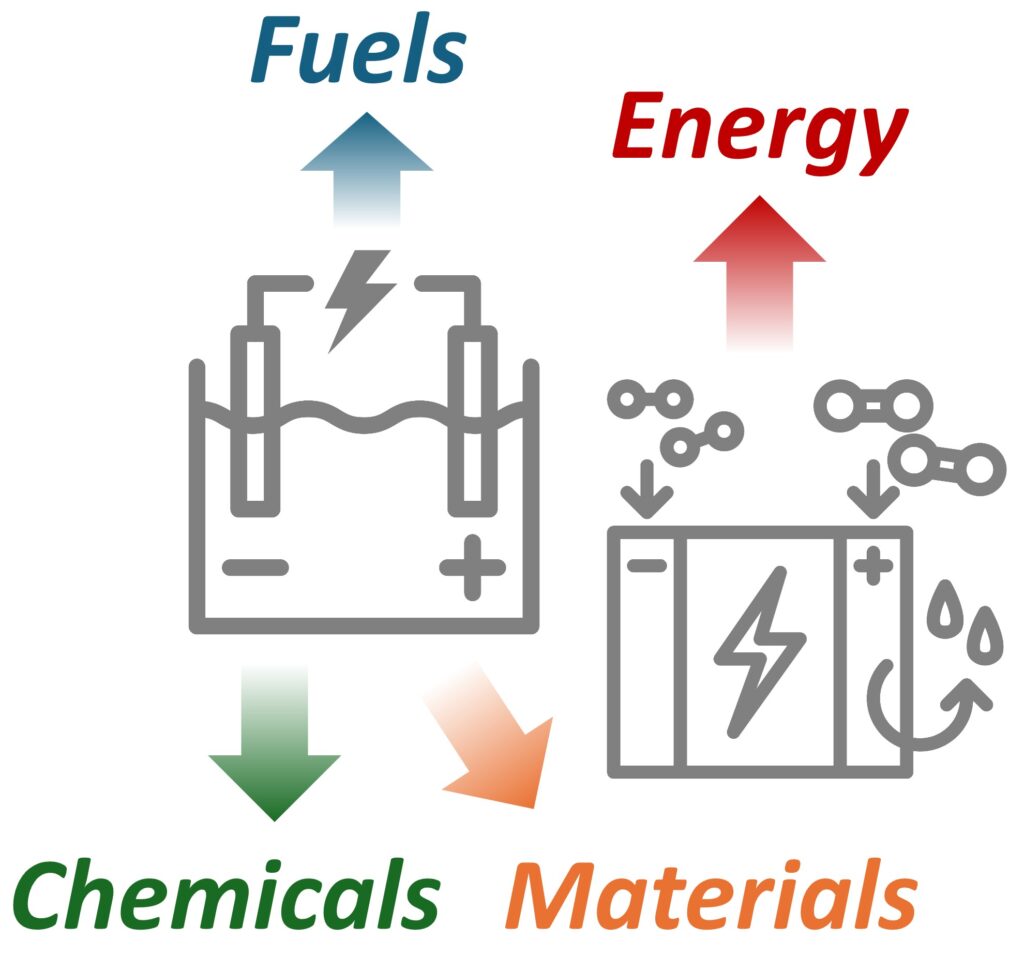
Figure 1. Various applications of electrochemical systems
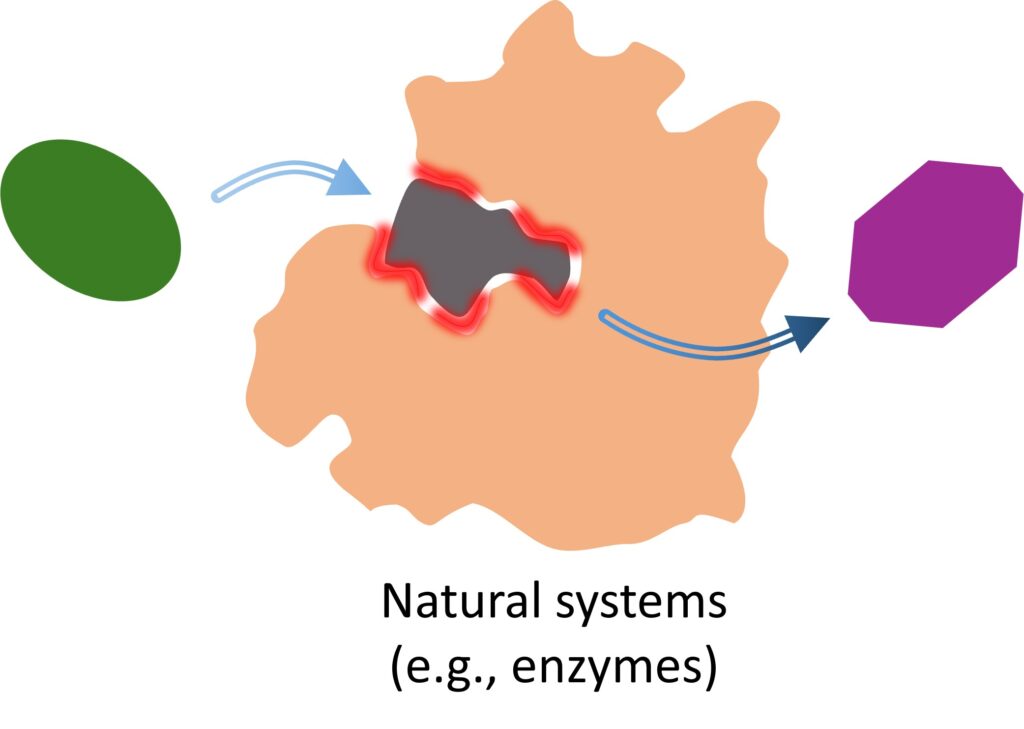
Figure 2. Chemical reactions facilitated
and guided by enzymes.
To realize such potential, focus should be on overcoming the fundamental challenges that currently limit the applicability of electrochemical reactions. The existing limitations mainly stem from insufficient molecular-level control over reaction pathways, which restricts not only reaction rates and selectivity but also the ability to precisely process large, complex reagents and products through multistep reactions. In contrast, nature’s molecular machinery, like enzymes, excels at this, featuring precisely configured reaction centers or pockets that uniquely bind the reagent through various interactions in its surroundings and orchestrate well-organized cascades of elementary reaction steps (Fig. 2).
At the Kim Lab, we focus on our fundamental understanding of the heterogeneity of electrochemical systems (Fig. 3), leading to unique materials and methods that improve the functionality and applicability of electrochemical processes and devices.
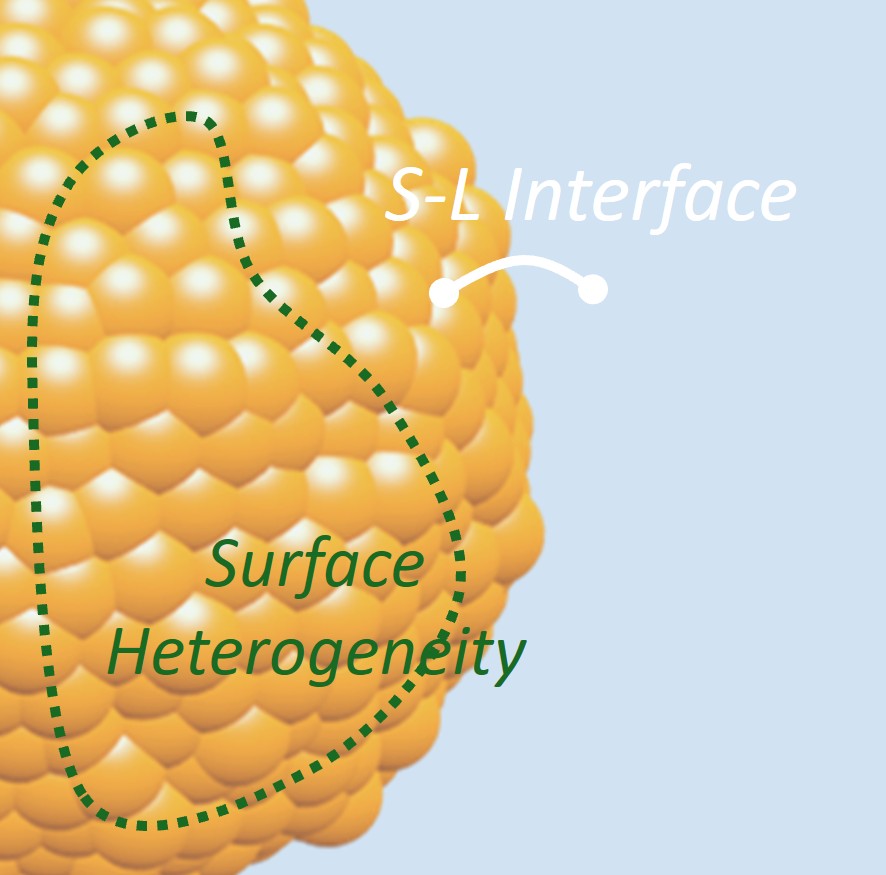
Figure 3. Heterogeneity in electrochemical systems.
Electrochemical reactions are heterogeneous reactions occurring at the interface of a solid and a liquid. Hence, what is present at the interfacial liquid, the liquid region in direct contact with a solid, is as important as the structure of the solid catalyzing the reaction (Fig. 4). This is an underexplored area with the potential to transform how electrochemical reactions are driven. Prior efforts have primarily focused on the control of material surfaces. By controlling both sides of the interface, the solid and liquid phase components can be synergistically combined to facilitate chemical reactions. This also means greater precision in guiding the reactions by coupling how reagent molecules interact with surfaces and the surrounding interfacial liquid, opening new possibilities. As part of our effort to better understand and tailor the properties of the interfacial liquid, we are particularly interested in the introduction and use of hydrophobes in the liquid media and the resulting hydrophobic effects that positively influence molecular interactions and reaction efficiency.
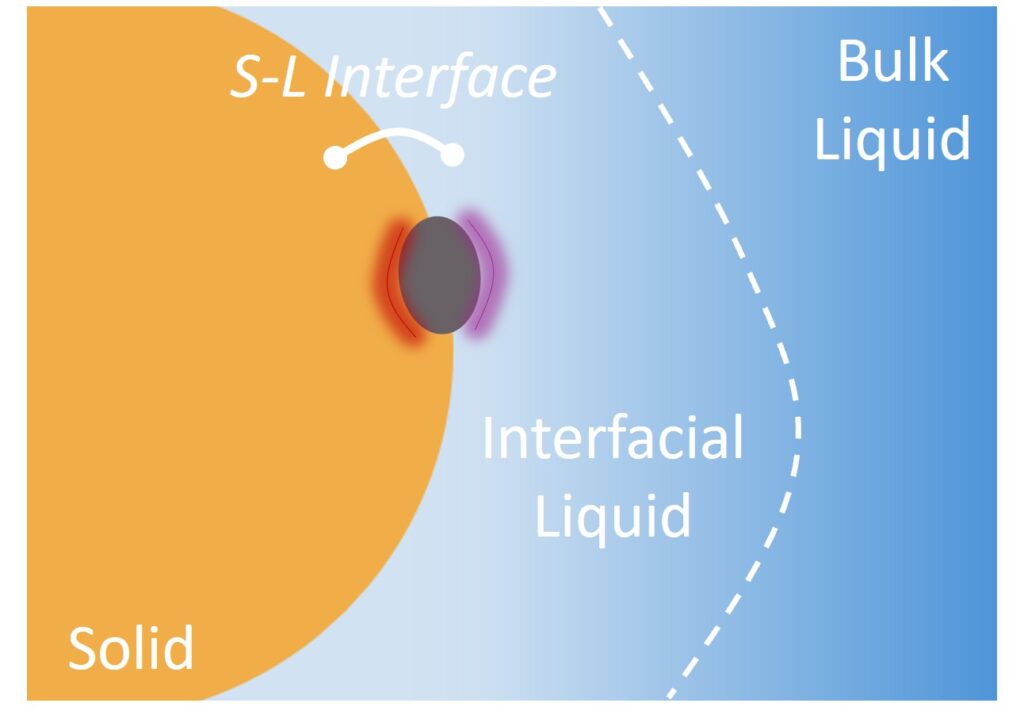
Figure 4. The role of interfacial liquid in molecular interactions.
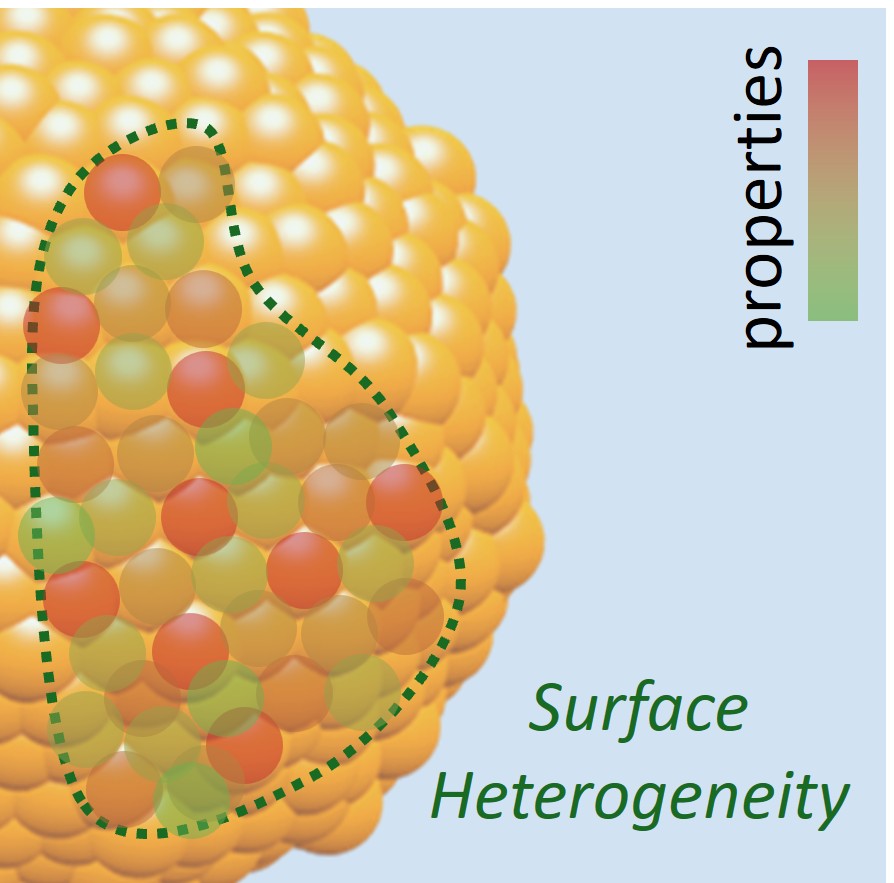
Figure 5. The heterogeneous nature of surfaces.
The chemical reaction outcomes result from the combined activity of numerous active sites on a solid material surface. The solid surfaces are often heterogeneous, featuring a diverse array of sites (composed of one or a few atoms per site) with differing properties for a reaction (Fig. 5). With existing technologies, we cannot fully understand the chemical activity landscape of material surfaces, probing activity at the individual site level and determining how the sites are distributed across material surfaces. However, with this foundational knowledge, it becomes possible to design solid materials that either combine an optimal variety of active site types on a single surface or feature a uniform population of the highest-performing site, maximizing overall reaction efficiency in ways that were previously out of reach. We are developing methods that can elucidate the surface chemical activity of materials down to the individual site level and the population of various site types based on activity and structure. Furthermore, we develop and validate novel synthetic strategies that enable precise control over material surfaces, allowing for the tailored selection of diverse active sites or the creation of surfaces composed entirely of a single site type. Through the lens of the unique capabilities of each active site and assembling them from the bottom up, we not only achieve greater control of the reaction on a macroscopic scale but also redefine the fundamental principles for designing chemically active solid materials. These efforts yield electrochemically active solids with significantly enhanced performance, far surpassing the existing state-of-the-art materials.
Heterogeneities can also be introduced at the level of individual active sites. Unlike thermodynamically stable, regular, and symmetric configurations, asymmetric and anisotropic active sites are less common and more challenging to create. However, these less favored configurations can unlock unique and enhanced catalytic properties, offering new avenues for chemical reactions. We explore novel approaches to construct libraries of asymmetric active sites, unlocking their broad potential for diverse applications across chemical catalysis and materials science.
Overall, we focus on advancing fundamental insights of heterogeneity at small scales (i.e., molecular, nanoscale) and having that knowledge translate into applicable strategies. Our advancements will drive the growth of electrochemical technologies across energy, fuel, chemical, and materials industries, transforming how these essential products are produced and utilized. This will enable greater efficiency, resilience, and security, ultimately enhancing the quality of life across society.
