The Control of Energy and Matter by
Electrochemically Advanced Surfaces and Interfaces at the Nanoscale
Chemical reactions driven by electrons (i.e., electrochemical reactions) can play a critical role in advancing sustainability. Using electricity produced from renewable sources, electrochemical reactions can be developed to establish carbon neutral or negative ways of storing and using energy as well as obtaining and processing resources to make essential products. To realize such potential, focus should be on overcoming the fundamental challenges that limit the efficiency and wide applicability of electrochemical processes.
The three fundamental challenges identified in our group are:
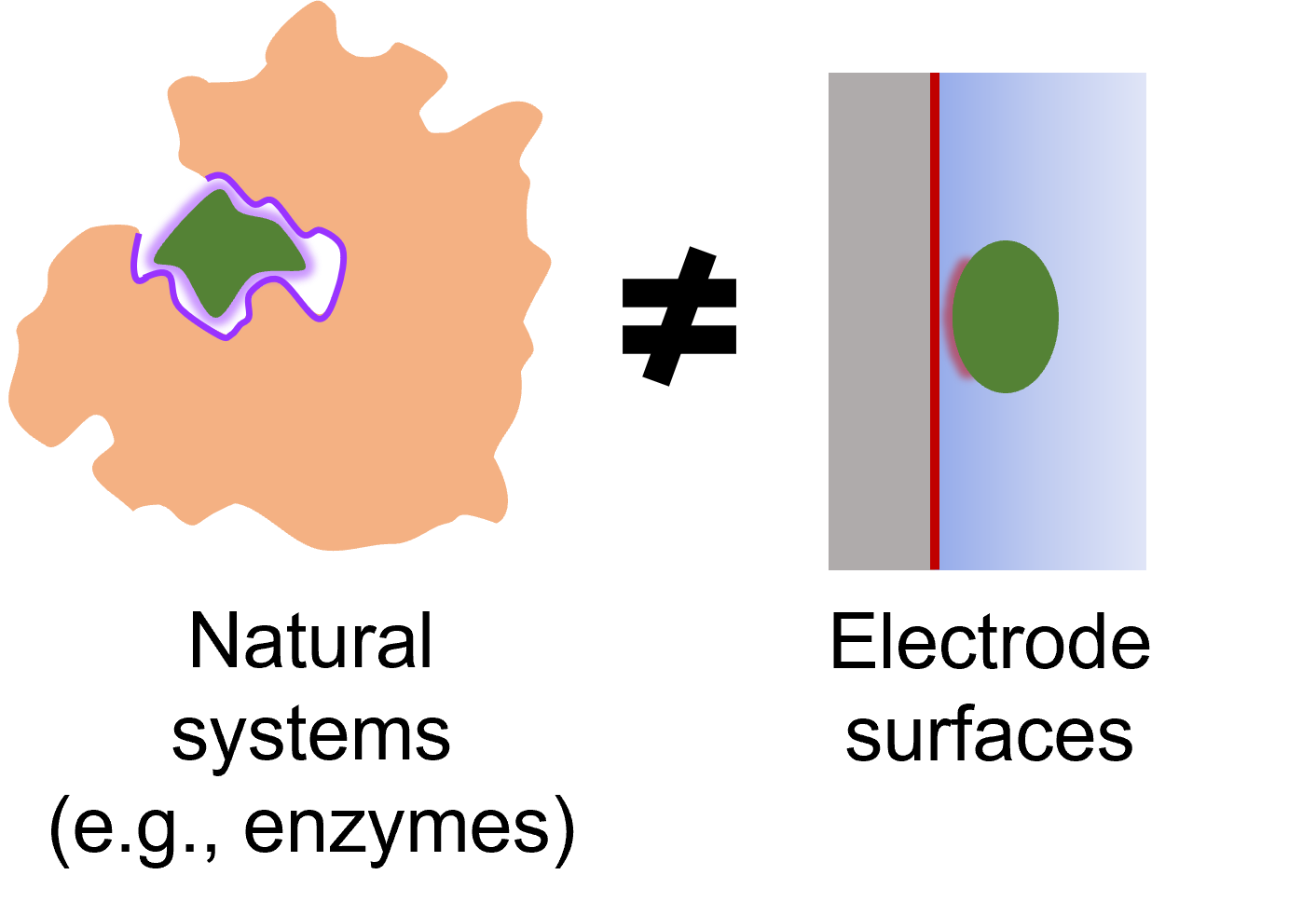
(1) Inability to achieve a multi-dimensional control of reactions. For instance, enzymes exhibit high activity/selectivity by the use of their catalytic pockets that uniquely bind the substrate through multiple interactions in its surroundings. However, electrochemical reactions driven solely by electrode surfaces cannot achieve the most ideal interactions with reactants and intermediates.
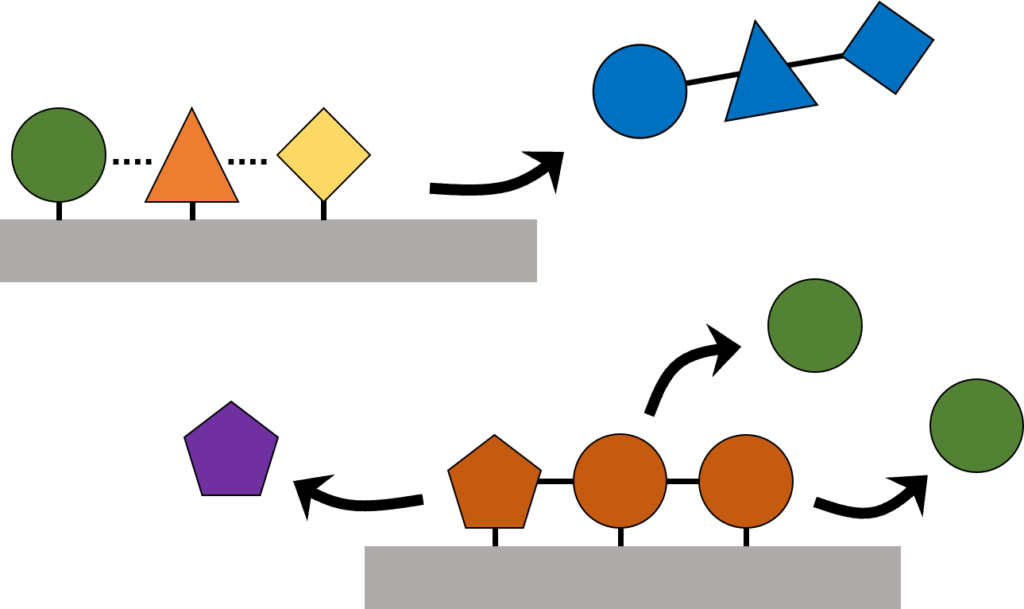
(2) Inability to enhance reaction complexity. Progress is restricted to certain reactions that begin with relatively simple molecules and generate a limited range of products. Advances are needed that can facilitate multistep reactions to generate complex products and chemically transform reactants of large molecular weights and complex structures.
(3) Limited understanding and control of solids in electrochemical environments. Efforts have primarily centered on electrochemical reactions of gases and liquids with solid materials merely used as means to achieve such reactions. How solid materials behave and form in electrochemical environments is a critical question that can improve existing reactions as well as open up new opportunities.
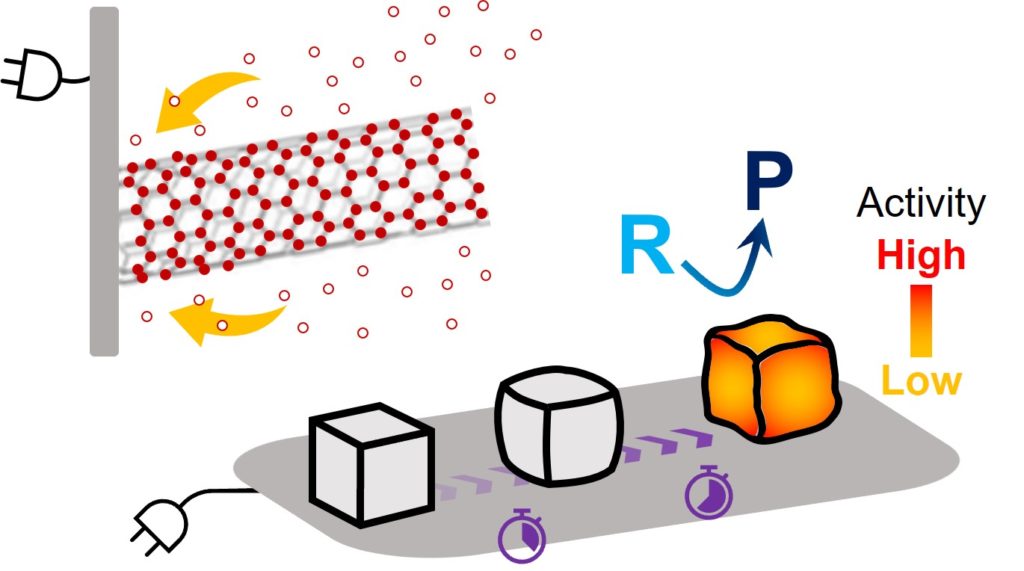
At Kim Lab, we aim to develop unique materials, processes, and methods that improve the functionality and applicability of electrochemically active surfaces and interfaces. Our activities focus on advancing fundamental insights at small scales (i.e., molecular, nanoscale) and having that knowledge transcend across length scales being translated into applicable strategies. Our progress will foster the growth of electrochemical applications in energy, chemicals, materials, and the environment and the transition to sustainability powered by renewable electricity.
